DRK42–Biologically Contaminated Aerosols Penetration Tester Operation Manual
Short Description:
1. Overview Refer to the following figures while reading this chapter. 1.1 Main Introduction 1.1.1 Standards ISO/DIS 22611 Clothing for protection against infectious agents-Test method for resistance to penetration by biologically contaminated aerosols. 1.1.2 Specifications l Aerosol generator: Atomizer l Exposure chamber : PMMA l Sample assembly : 2, stainless steel l Vacumm pump : Up to 80kpa l Dimension : 300mm*300mm*300mm l Power supply : 220V 50-60Hz l Machine Dimension: 46cm×9...
1. Overview
Refer to the following figures while reading this chapter.
1.1 Main Introduction
1.1.1 Standards
ISO/DIS 22611 Clothing for protection against infectious agents-Test method for resistance to penetration by biologically contaminated aerosols.
1.1.2 Specifications
l Aerosol generator: Atomizer
l Exposure chamber : PMMA
l Sample assembly : 2, stainless steel
l Vacumm pump : Up to 80kpa
l Dimension : 300mm*300mm*300mm
l Power supply : 220V 50-60Hz
l Machine Dimension: 46cm×93cm×49cm(H)
l Net Weight:35kg
2. USE OF THE EQUIPMENT
2.1 Preparation
Put the three parts in the biosafety cabinet. Check each parts of the test machine and make sure all the parts working well and connecting well.
Cutting eight samples as 25mm diameter circles.
Prepare an overnight culture of Staphylococcus aureus by aseptic transfer of the bacterium from nutrient agar (stored at 4±1℃) into nutrient broth and incubation at 37±1℃ on an orbital shaker.
Dilute the culture into an appropriate volume of sterile isotonic saline to give a final bacterial count of approximately 5*107 cells cm-3 using Thoma bacterial counting chamber.
Fill the culture above into the atomizer. Liquid level is between upper level and lower level.
2.2 Operation
Install the sample assembly. Put silicone washer A, test fabric, silicone washer B, membrane, wire support on the open lid, cover with the base.
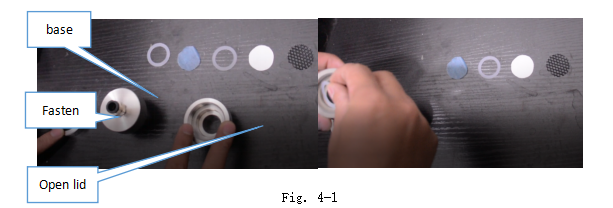
Install the other sample assembly without sample.
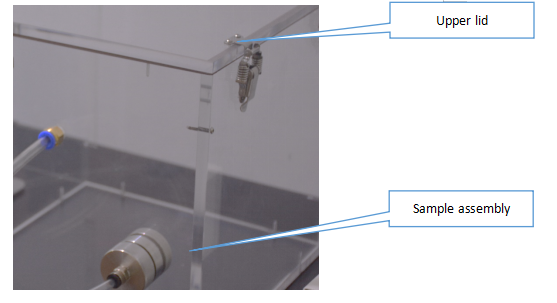
Open the upper lid of test chamber.
Install the sample assembly with sample and assembly without sample by Fasten of Fig. 4-1.
Make sure all the tubes connected well.
Connect the compressed air to compressed air adjust.
Apply air at flow of 5L/min by adjusting the flow meter to the atomizer and start generating the aerosol.
After 3 mins activate the vacumm pump. Set it as 70kpa.
After 3 mins, turn off the air to atomizer, but leave vacuum pump running for 1 min.
Turn off the vaccum pump.
Remove the sample assemblies from the chamber. And aseptically transfer the 0.45um membranes to universal bottles containing 10ml sterile isotonic saline.
Extract by shaking for 1 min. And make serial dilutions with sterile saline. (10-1, 10-2, 10-3, and 10-4)
Plate out 1ml aliquots of each dilution in duplicate using nutrient agar.
Incubate the plates overnight at 37±1℃ and express the results using the ratio of the background bacterial count to the number of bactria passed throug the test sample.
Make four determinations on each fabric type or fabric condition.
3. MAINTENANCE
As with all electrical equipment, this unit must be used correctly and maintenance and inspections must be performed at regular intervals. Such precautions will guarantee the safe and efficient functioning of the equipment.
Periodic maintenance consists of inspections made directly by the test operator and/or by the authorized service personnel.
Maintenance to the equipment is responsibility of the purchaser and must be performed as stated by this chapter.
Failing to perform the recommended maintenance actions or maintenance performed by unauthorized people can void the warranty.
1. The machine must be checked to prevent leakage of connections before tests;
2. Moving the machine is forbidden when using it;
3. Choose the corresponding power supply and voltage. Don’t too high to avoid burning device;
4. Please contact us in order to handle in time when the machine is out of order;
5. It must have a good ventilation environment when the machine works;
6. Cleaning the machine after test every time;
|
Action |
Who |
When |
| Check to ensure that there is no external damage to the machine, which could jeopardize the safety of use. | Operator | Before every working session |
| Cleaning the machine | Operator | At end of each test |
| Checking leakage of connections | Operator | Before test |
| Checking status and functioning of the buttons, operator’s command. | Operator | Weekly |
| Checking power cord properly attached or not. | Operator | Before test |

SHANDONG DRICK INSTRUMENTS CO.,LTD
Company Profile
Shandong Drick Instruments Co., Ltd, is mainly engaged in the research and development, manufacturing and sales of testing instruments.
The company established in 2004.
Products are used in scientific research units, quality inspection institutions, universities, packaging, paper, printing, rubber and plastics, chemicals, food, pharmaceuticals, textiles, and other industries.
Drick pays attention to talent cultivation and team building, adhering to the development concept of professionalism, dedication.pragmatism, and innovation.
Adhering to the customer-oriented principle, solve the most urgent and practical needs of customers, and provide first-class solutions to customers with high-quality products and advanced technology.